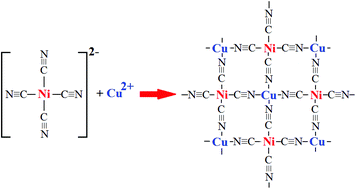
Chemistry and structure by design: ordered CuNi(CN)4 sheets with copper(ii) in a square-planar environment - Dalton Transactions (RSC Publishing)
Ni(CN)4]^2- is diamagnetic, while [Ni(CN)4]^2- is paramagnetic, explain using crystal field theory. - Sarthaks eConnect | Largest Online Education Community
Deduce the structures of [NiCl4]^2- and [Ni(CN)4]^2- considering the hybridisation of the metal ion. - Sarthaks eConnect | Largest Online Education Community
![The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com](https://homework.study.com/cimages/multimages/16/cms53022462554618608855.jpg)
The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com
![what is the geometry of [Ni(CN)6]4-,,,can i have its structure ,,orbital structure - Chemistry - Coordination Compounds - 6885308 | Meritnation.com what is the geometry of [Ni(CN)6]4-,,,can i have its structure ,,orbital structure - Chemistry - Coordination Compounds - 6885308 | Meritnation.com](https://img-nm.mnimgs.com/img/study_content/content_ck_images/images/NiCN6(3).PNG)
what is the geometry of [Ni(CN)6]4-,,,can i have its structure ,,orbital structure - Chemistry - Coordination Compounds - 6885308 | Meritnation.com
![Diamagnetic - Paramagnetic - Ni(CO)4, [Ni(CN)4]2- and [NiCl4]2- IIT JEE ... | Chemistry lessons, Chemistry, Chemistry degree Diamagnetic - Paramagnetic - Ni(CO)4, [Ni(CN)4]2- and [NiCl4]2- IIT JEE ... | Chemistry lessons, Chemistry, Chemistry degree](https://i.pinimg.com/originals/a2/20/10/a22010863c43298057a81f0eb09dd692.jpg)
Diamagnetic - Paramagnetic - Ni(CO)4, [Ni(CN)4]2- and [NiCl4]2- IIT JEE ... | Chemistry lessons, Chemistry, Chemistry degree
![The structure of [NiCl4]^2 - and [Ni(CN)4]^2 - considering the hybridisation of the metal ion are respectively: The structure of [NiCl4]^2 - and [Ni(CN)4]^2 - considering the hybridisation of the metal ion are respectively:](https://haygot.s3.amazonaws.com/questions/1155452_190692_ans_2bceb3b98765436db10c686499b5ef87.png)
The structure of [NiCl4]^2 - and [Ni(CN)4]^2 - considering the hybridisation of the metal ion are respectively:
![The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com](https://homework.study.com/cimages/multimages/16/cms63713049016565485752.jpg)
The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com
![Synthesis and molecular structure of [Cu(NH3)4][Ni(CN)4]: A missing piece in the [Cu(NH3)n][Ni(CN)4] story - ScienceDirect Synthesis and molecular structure of [Cu(NH3)4][Ni(CN)4]: A missing piece in the [Cu(NH3)n][Ni(CN)4] story - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022286015300624-fx1.jpg)
Synthesis and molecular structure of [Cu(NH3)4][Ni(CN)4]: A missing piece in the [Cu(NH3)n][Ni(CN)4] story - ScienceDirect
![NiCl4] 2- is paramagnetic while [Ni(CN) 4 ] 2- is diamagnetic | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium NiCl4] 2- is paramagnetic while [Ni(CN) 4 ] 2- is diamagnetic | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:578/0*WbYEQKZnf6lHveOA.jpg)
NiCl4] 2- is paramagnetic while [Ni(CN) 4 ] 2- is diamagnetic | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Explain hybridisation, geometry and magnetic property of [Ni(CN)4]^2 - ion using Valence Bond Theory(VBT). [Atomic number of Ni is 28 ]. Explain hybridisation, geometry and magnetic property of [Ni(CN)4]^2 - ion using Valence Bond Theory(VBT). [Atomic number of Ni is 28 ].](https://haygot.s3.amazonaws.com/questions/874625_947137_ans_e945a0328151431c8601af56eaa7dcb3.png)
![The geometry of [Ni(CN)4]^2 - and [NiCl4]^2 - ions are : The geometry of [Ni(CN)4]^2 - and [NiCl4]^2 - ions are :](https://d1hj4to4g9ba46.cloudfront.net/questions/345819_212776_ans_5a2a220d536a4adebbfad43e7ddf74b5.png)

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure -Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure -Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)
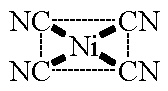
![Explain the structure of [Ni(CN)4]²- on the basis of Valence bond theory - Brainly.in Explain the structure of [Ni(CN)4]²- on the basis of Valence bond theory - Brainly.in](https://hi-static.z-dn.net/files/d36/7c6355211a67367b10a284b545b50f8f.jpg)
![Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube](https://i.ytimg.com/vi/r_C4yyTUSjM/maxresdefault.jpg)
![coordination compounds - Electronic configuration in [Ni(CN)4]2- - Chemistry Stack Exchange coordination compounds - Electronic configuration in [Ni(CN)4]2- - Chemistry Stack Exchange](https://i.stack.imgur.com/znHnV.png)
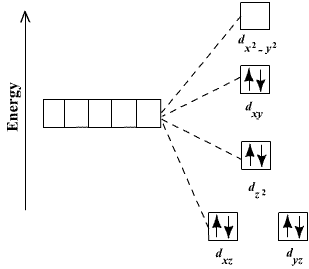
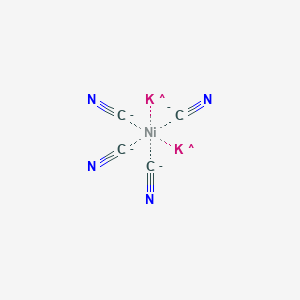
![Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with - askIITians Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with - askIITians](https://files.askiitians.com/cdn1/cms-content/common/www.askiitians.comonlinetestforumsimages204-1478_thuaug1404-05-56.jpg.jpg)
![Hybridization and geometry of [Ni(CN)4]^2 - are: Hybridization and geometry of [Ni(CN)4]^2 - are:](https://haygot.s3.amazonaws.com/questions/623286_597576_ans_83f06d1a64a9465c875f91d8efb8e27d.png)
